At PamGene we are dedicated to support physicians and scientists in finding solutions and answers to improve and optimize patient treatment and understand diseases. Our multiplex kinase activity profiling technology reveals the bigger picture of cellular signaling and provides mechanistic insights needed to fully understand diseases and cell alterations.
At PamGene we are dedicated to support physicians and scientists in finding solutions and answers to improve and optimize patient treatment and understand diseases. Our multiplex kinase activity profiling technology reveals the bigger picture of cellular signaling and provides mechanistic insights needed to fully understand diseases and cell alterations.
At PamGene we are dedicated to support physicians and scientists in finding solutions and answers to improve and optimize patient treatment and understand diseases. We have developed a robust and unique microarray technology for multiplex kinase activity profiling and offer dedicated assay services for biomarker discovery, patient stratification and therapy selection for oncological diseases. PamGene is a Dutch biotechnology company founded in 2000 and with headquarters in Den Bosch, the Netherlands.

At PamGene we are dedicated to support physicians and scientists in finding solutions and answers to improve and optimize patient treatment and understand diseases. We have developed a robust and unique microarray technology for multiplex kinase activity profiling and offer dedicated assay services for biomarker discovery, patient stratification and therapy selection for oncological diseases. PamGene is a Dutch biotechnology company founded in 2000 and with headquarters in Den Bosch, the Netherlands.

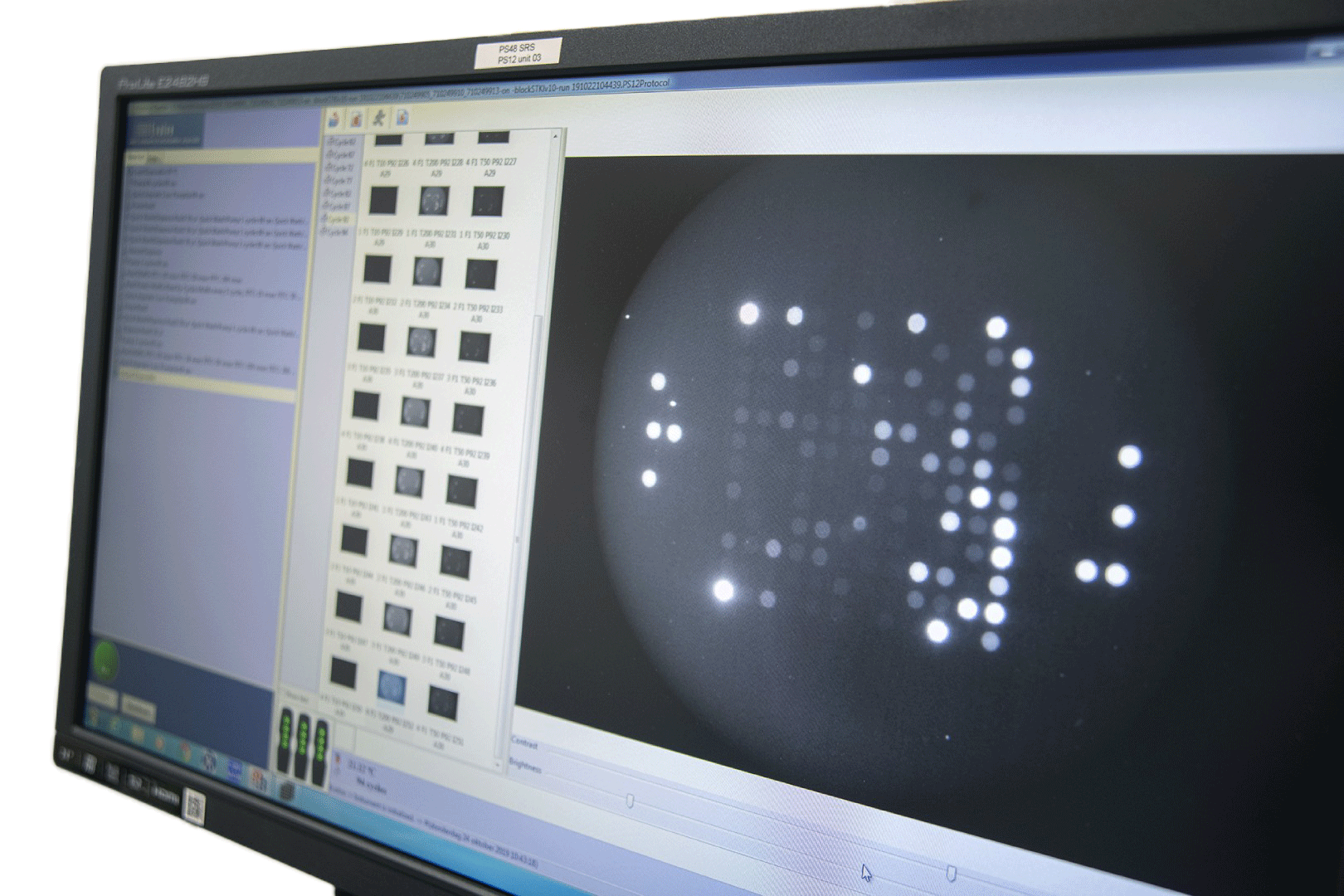
Rapid and non-invasive measurement of real-time cellular kinome activity results in comprehensive and accurate data. Our technology is used in clinical, translational and pre-clinical settings for various disease areas to gain insights in discriminative drug responses, analyzing and optimizing kinase inhibitors; and discovery of novel biomarkers, targets and pathways.
PamGene provides access to its technology through offering Contract Research Services and Support from our facilities in ‘s-Hertogenbosch, the Netherlands. Our Diagnostic Assay Services (DAS) facility is ISO13485 compliant and provides in-vitro diagnostic assay services for the prediction of therapy response for oncological diseases.
Rapid and non-invasive measurement of real-time cellular kinome activity results in comprehensive and accurate data. Our technology is used in clinical, translational and pre-clinical settings for various disease areas to gain insights in discriminative drug responses, analyzing and optimizing kinase inhibitors; and discovery of novel biomarkers, targets and pathways.
PamGene provides access to its technology through offering Contract Research Services and Support from our facilities in ‘s-Hertogenbosch, the Netherlands. Our Diagnostic Assay Services (DAS) facility is ISO13485 compliant and provides in-vitro diagnostic assay services for the prediction of therapy response for oncological diseases.

PamGene’s team of scientists, analysts and technicians build on a long-standing experience in kinase science. Our state-of-the-art infrastructure, including our in-house array production and bioinformatics support, provides our clients with comprehensive data to support improvement of patient treatment.

PamGene’s team of scientists, analysts and technicians build on a long-standing experience in kinase science. Our state-of-the-art infrastructure, including our in-house array production and bioinformatics support, provides our clients with comprehensive data to support improvement of patient treatment.

