Are you interested in gaining valuable insights for your lead kinase inhibitor?
Are you interested in gaining valuable insights for your lead kinase inhibitor?
Are you interested in gaining valuable insights for your lead kinase inhibitor? PamGene is operating on the cutting edge of next generation kinase science and high-tech to support scientists and companies in discovering, studying and optimizing kinase inhibitors. Building on over 15 years of experience, we are able to go beyond recombinant kinase screening to provide you with a better understanding of your compound’s:
Are you interested in gaining valuable insights for your lead kinase inhibitor? PamGene is operating on the cutting edge of next generation kinase science and high-tech to support scientists and companies in discovering, studying and optimizing kinase inhibitors. Building on over 15 years of experience, we are able to go beyond recombinant kinase screening to provide you with a better understanding of your compound’s:
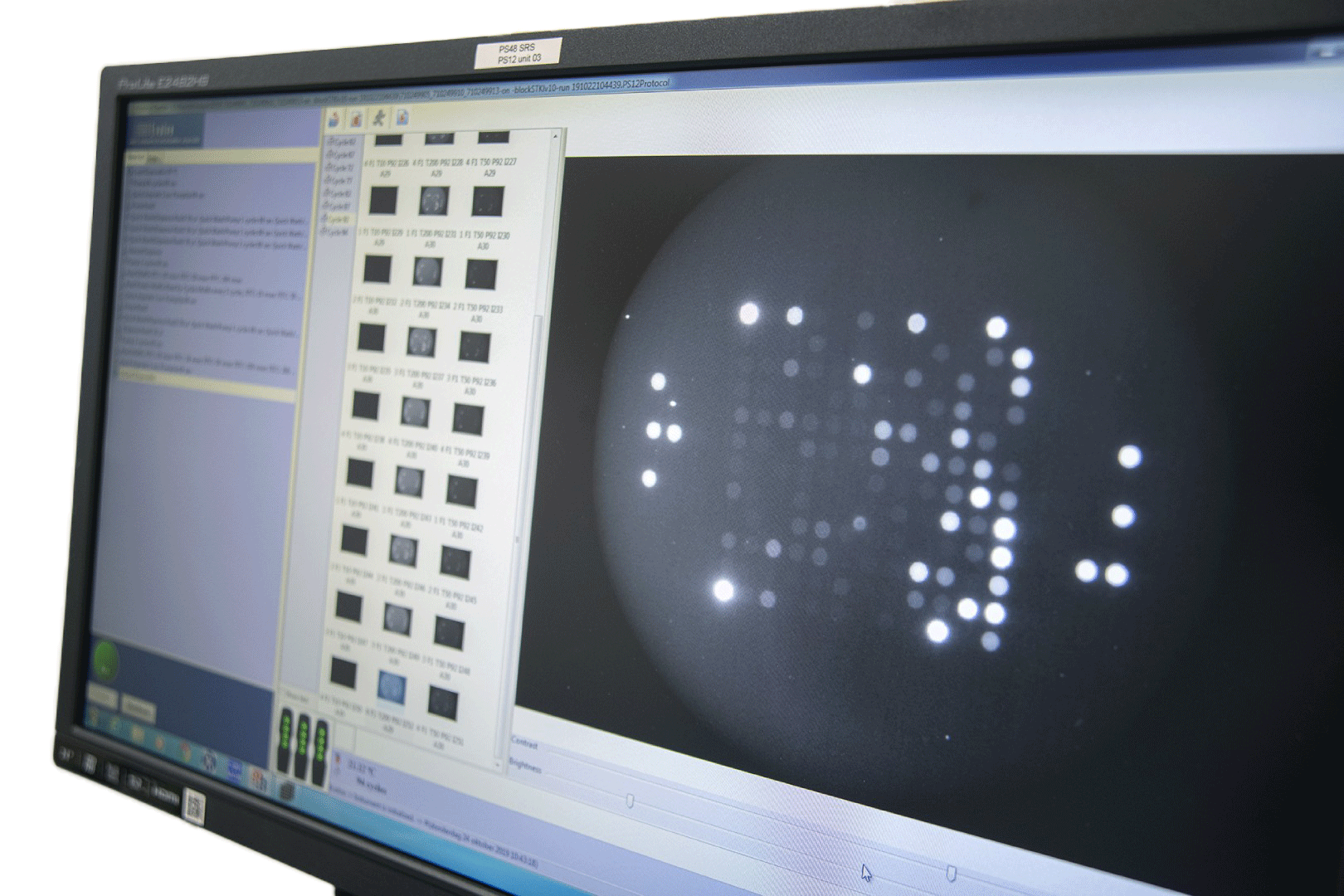
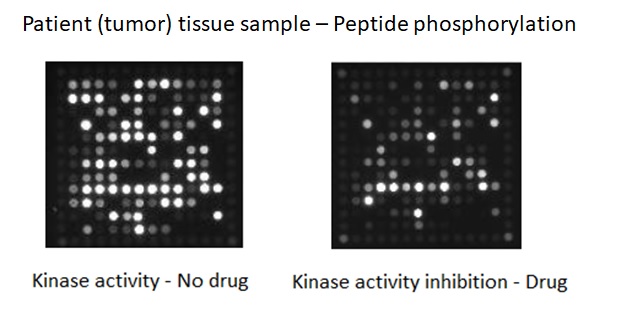
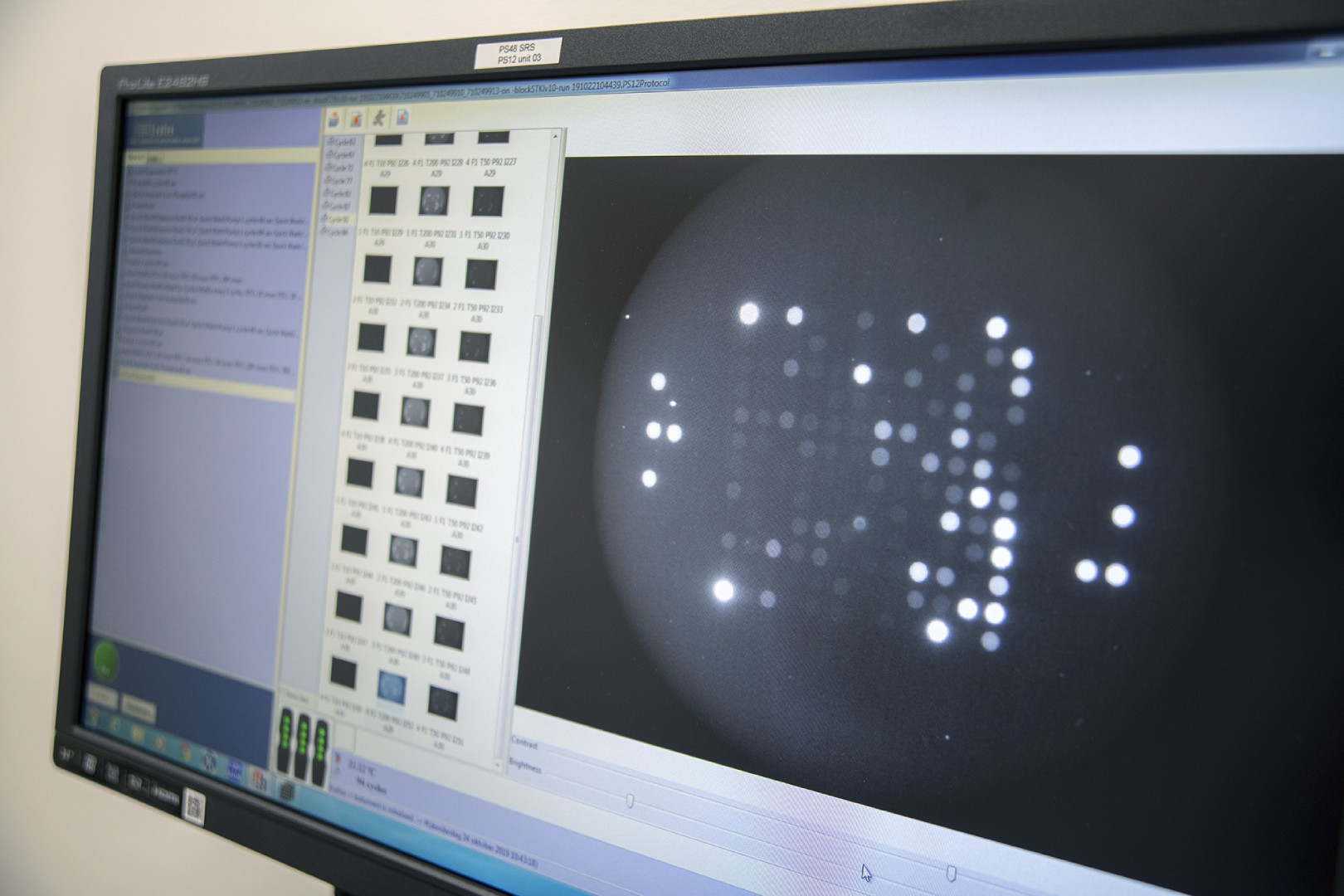
Kinases are the most intensively studied protein targets. We have developed a unique method to understand them. Our sensitive platform uses lysates obtained from only a few thousand cells or small amounts (2ug) of animal, xenografts or human tissues and cells to simultaneously determine the activity of all kinases present in these lysates.
This is accomplished by incubating the lysates across 196 to 144 tyrosine or serine/threonine kinase peptide substrates immobilized on the PamChip® microarray platform. Our 3D microarrays are spotted with peptides (i.e. phosphosites) that represent kinase targets. Kinases present in the lysates will phosphorylate the peptide substrates which are detected using fluorescently labelled antibodies. The phosphosites on the PamChips are human-derived and there is significant overlap with other organisms.
Based on current online knowledge, we compiled a comprehensive, integrated database (DB) of potential kinases that are linked to the peptides on the PamChips. This corresponds to ~350 unique kinases in literature, covering the majority of the kinome.
Kinases are the most intensively studied protein targets. We have developed a unique method to understand them. Our sensitive platform uses lysates obtained from only a few thousand cells or small amounts (2ug) of animal, xenografts or human tissues and cells to simultaneously determine the activity of all kinases present in these lysates.
This is accomplished by incubating the lysates across 196 to 144 tyrosine or serine/threonine kinase peptide substrates immobilized on the PamChip® microarray platform. Our 3D microarrays are spotted with peptides (i.e. phosphosites) that represent kinase targets. Kinases present in the lysates will phosphorylate the peptide substrates which are detected using fluorescently labelled antibodies. The phosphosites on the PamChips are human-derived and there is significant overlap with other organisms.
Based on current online knowledge, we compiled a comprehensive, integrated database (DB) of potential kinases that are linked to the peptides on the PamChips. This corresponds to ~350 unique kinases in literature, covering the majority of the kinome.

Thanks to our unique and proven technology we will provide you with robust measurements, which can be applied in numerous of applications bringing your insights and research to the next level.

Thanks to our unique and proven technology we will provide you with robust measurements, which can be applied in numerous of applications bringing your insights and research to the next level.

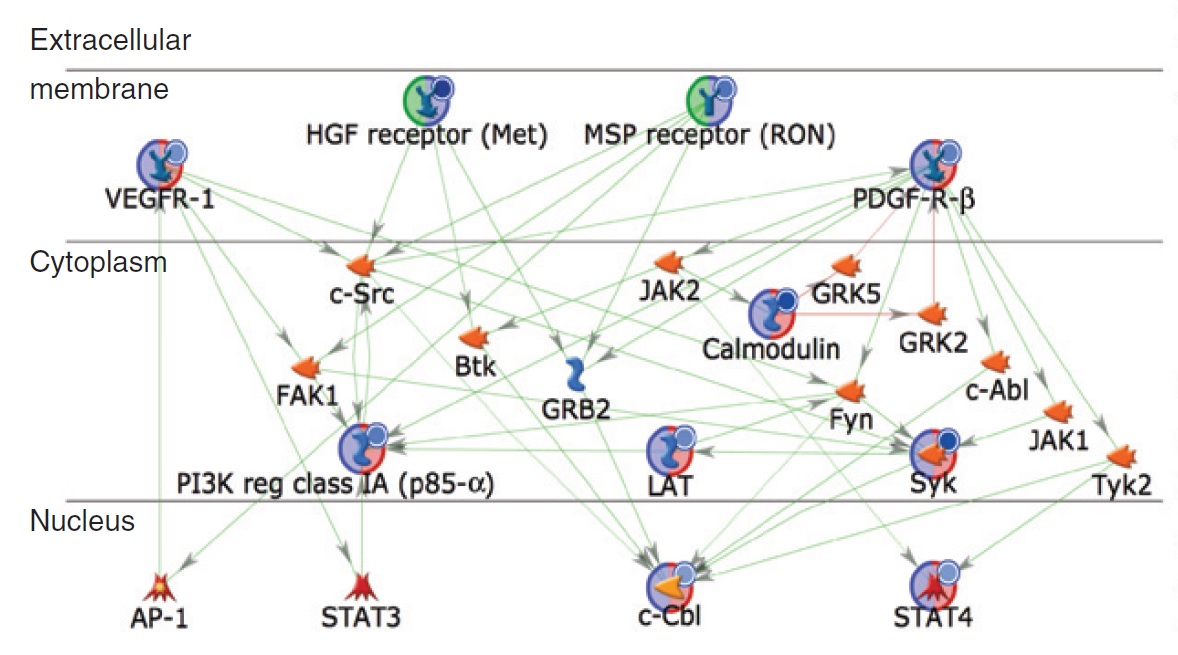
We have over 15 years of experience in the field of kinase science and contributed to over 100+ publications with top European, North American and Japanese Institutes.
To learn how our services contributed to different studies, request access to our case report.
de Korte T, Nacken P, Pijnenburg D, Ruijtenbeek R, Stevenhagen F, Wilbers R, den Hartogh S, Groten J, Braam S and Vlaming M
Journal of Pharmacological and Toxicological Methods. 88. 236-237. 10.1016. (2017)
Blythe NM, Muraki K, Ludlow MJ, Stylianidis V, Gilbert HTJ, Evans EL, Cuthbertson K, Foster R, Swift J, Li J, Drinkhill MJ, van Nieuwenhoven FA, Porter KE, Beech DJ, Turner NA.
J Biol Chem. Oct 4. (2019)
We have over 15 years of experience in the field of kinase science and contributed to over 100+ publications with top European, North American and Japanese Institutes.
To learn how our services contributed to different studies, request access to our case report.
de Korte T, Nacken P, Pijnenburg D, Ruijtenbeek R, Stevenhagen F, Wilbers R, den Hartogh S, Groten J, Braam S and Vlaming M
Journal of Pharmacological and Toxicological Methods. 88. 236-237. 10.1016. (2017)
Steen NV, Potze L, Giovannetti E, Cavazzoni A, Ruijtenbeek R, Rolfo C, Pauwels P, Peters GJ.
Am J Cancer Res. Apr 1;7(4):816-830. eCollection 2017.
