Kinases play a crucial role in immune checkpoint regulation and therefore can predict a patient’s response before immune checkpoint inhibitor (ICI) therapy is started. The response to ICI therapy is driven by an individual patient’s kinase activity profile and can give key information to clinicians in support of treatment decisions.
72 melanoma and 65 NSCLC patients were included in the interim-analysis for the ongoing multicenter prospective cohort study. Significant differences between tyrosine kinase activity profiles of the progression versus no progression group were observed for both the melanoma and NSCLC cohort showing that this form of profiling serves as a predictive biomarker for tumor response to ICIs, with a Correct Classification Rate of 71% for the melanoma cohort and 70% for the NSCLC cohort.
Predictive performance may improve when combined with existing biomarkers: This is achieved when tyrosine kinase activity profiling is combined with low tumor PD-L1 expression in NSCLC patients, as depicted on the right. Validation of the results in an independent patient cohort is currently ongoing.
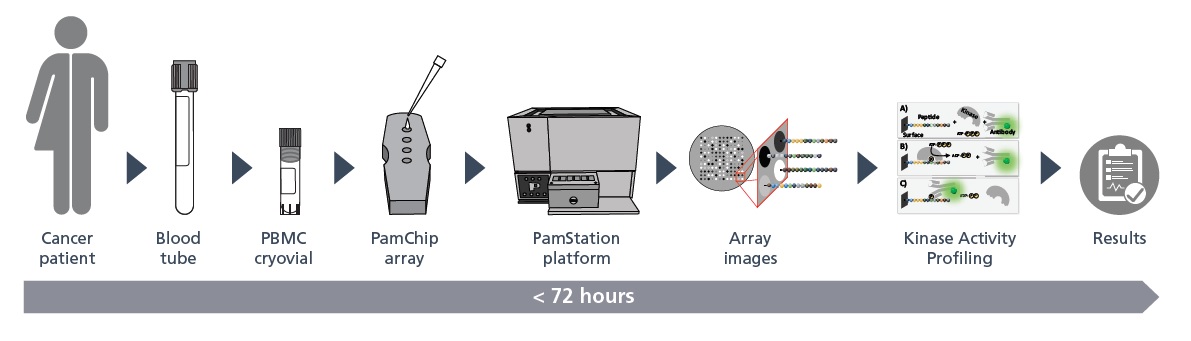

PamGene’s Diagnostic Assay Services Facility performs the IOpener™ test as IVD assay service to help targeting the most appropriate treatment for individual melanoma and lung cancer patients. The IOpener™ test is currently available as in-house developed test. Since July 2022, the IOpener®-melanoma and the IOpener®-NSCLC tests have been registered as CE-IVDs under the in-vitro diagnostic medical device Directive (98/79/EG) by the Dutch Ministry of Health, Welfare and Sport. The IOpener™ tests are currently available as in-house developed tests and approved for clinical studies and for use in diagnostic procedures.
The IOpener™ tests are currently available as in-house developed test via PamGene Diagnostic Assay Services Facility. We closely collaborate with clinical professionals to extend our network in Europe and US. Please contact us if you are interested to learn more.
The IOpener™ tests are currently available as in-house developed tests for melanoma and NSCLC. Please contact us or your treating physician if you are interested to learn more.